Sleep medication dependency represents a complex clinical challenge that affects millions of individuals worldwide who rely on hypnotic medications for chronic insomnia management. This comprehensive review examines the neurobiological mechanisms underlying dependency development, explores the spectrum of withdrawal phenomena, and provides evidence-based strategies for safe medication discontinuation. The analysis encompasses various classes of sleep medications, including benzodiazepines, non-benzodiazepine hypnotics, and newer agents such as dual orexin receptor antagonists, with particular attention to their distinct dependency profiles and withdrawal characteristics. Contemporary research reveals significant differences in dependency potential among medication classes, with newer agents demonstrating reduced risk profiles compared to traditional benzodiazepines. The review synthesizes current understanding of tolerance mechanisms, receptor adaptation processes, and the complex interplay between psychological and physiological dependency factors. Safe discontinuation strategies require individualized approaches incorporating gradual tapering protocols, comprehensive withdrawal symptom management, and integration of non-pharmacological interventions such as cognitive behavioral therapy for insomnia. Healthcare providers must balance the therapeutic benefits of long-term hypnotic use against potential dependency risks while implementing evidence-based discontinuation protocols that minimize withdrawal distress and prevent rebound phenomena.
Introduction
The prevalence of chronic insomnia and the consequent use of hypnotic medications have increased substantially over recent decades, creating unprecedented challenges in sleep medicine practice. While these medications provide essential therapeutic benefits for individuals suffering from persistent sleep disturbances, their potential for dependency development represents a significant clinical concern that requires careful consideration and management. Sleep medication dependency encompasses a complex spectrum of physiological and psychological adaptations that can complicate treatment discontinuation and contribute to long-term healthcare challenges.
Understanding the mechanisms underlying sleep medication dependency requires examination of multiple interconnected factors, including neurobiological adaptations, psychological reliance patterns, and the intricate relationship between medication effects and underlying sleep pathophysiology. The development of dependency involves progressive neuroadaptive changes that alter brain function in ways that extend beyond the original therapeutic targets, creating conditions that perpetuate medication reliance and complicate discontinuation efforts.
The clinical significance of sleep medication dependency extends beyond individual patient concerns to encompasses broader public health implications, including healthcare utilization patterns, medication costs, and the potential for adverse events associated with long-term use. Healthcare systems worldwide face increasing pressure to develop comprehensive approaches that balance the legitimate therapeutic needs of patients with chronic insomnia against the risks associated with prolonged hypnotic medication use.
Contemporary research has revealed important distinctions among different classes of sleep medications regarding their dependency potential, withdrawal characteristics, and optimal discontinuation strategies. These findings have important implications for clinical practice, suggesting that individualized approaches to medication selection and discontinuation planning may significantly improve patient outcomes while minimizing dependency-related complications.
Neurobiological Mechanisms of Dependency Development
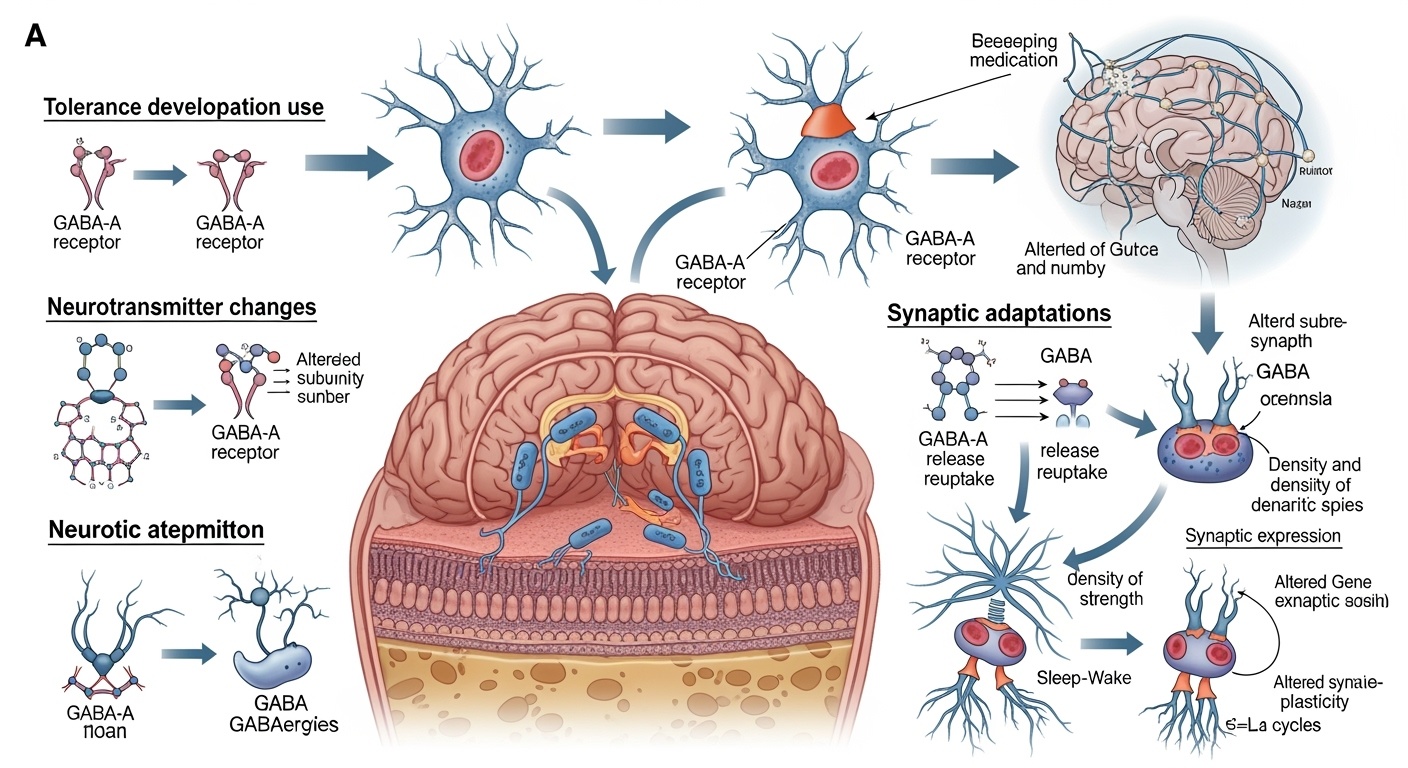
The development of sleep medication dependency involves complex neuroadaptive processes that fundamentally alter brain function and create conditions that perpetuate medication reliance. Understanding these mechanisms requires examination of how chronic exposure to hypnotic medications affects neurotransmitter systems, receptor function, and neural network activity patterns that regulate sleep-wake cycles and related physiological processes.
Gamma-aminobutyric acid neurotransmission serves as the primary target for most traditional sleep medications, particularly benzodiazepines and non-benzodiazepine hypnotics. Chronic exposure to these medications leads to progressive downregulation of GABA-A receptors and alterations in receptor subunit composition that fundamentally change the brain’s response to both endogenous and exogenous GABA-ergic signaling. This neuroadaptive process represents the brain’s attempt to maintain homeostatic balance in the presence of persistent pharmacological enhancement of inhibitory neurotransmission.
The molecular mechanisms underlying tolerance development involve multiple cellular processes that occur simultaneously across different brain regions. Receptor internalization and degradation reduce the total number of available GABA-A receptors at synaptic sites, while changes in receptor phosphorylation status alter the functional properties of remaining receptors. These adaptations result in progressively diminished medication efficacy, requiring dose escalation to maintain therapeutic effects and creating conditions that predispose to dependency development.
Neuroplastic changes extend beyond GABA-ergic systems to encompass alterations in glutamatergic neurotransmission, which provides excitatory balance to inhibitory GABA signaling. Chronic hypnotic exposure leads to compensatory upregulation of glutamate receptors and enhanced glutamatergic neurotransmission that serves to counteract medication-induced inhibition. This excitatory rebound becomes problematic during medication discontinuation, contributing to withdrawal symptoms and rebound insomnia phenomena.
The temporal dynamics of neuroadaptation vary significantly among different medication classes and individual patients, influencing both the rate of dependency development and the complexity of withdrawal management. Benzodiazepines with longer half-lives may produce more gradual adaptive changes compared to shorter-acting agents, while individual genetic variations in drug metabolism and receptor expression patterns contribute to significant inter-individual differences in dependency susceptibility.
Epigenetic modifications represent an emerging area of understanding regarding long-term neuroadaptive changes associated with chronic hypnotic use. These modifications can alter gene expression patterns that persist beyond the period of active medication exposure, potentially contributing to protracted withdrawal symptoms and increased vulnerability to relapse following successful discontinuation.
The involvement of dopaminergic reward pathways in sleep medication dependency remains less well understood compared to other substance dependencies, but emerging evidence suggests that hypnotic medications may influence reward processing in ways that contribute to psychological dependence. These effects may be particularly relevant for medications that produce subjective experiences of relaxation or euphoria beyond their primary sleep-promoting effects.
Classifications and Dependency Profiles of Sleep Medications
Sleep medications encompass diverse pharmacological classes with distinct mechanisms of action, therapeutic profiles, and dependency potentials that significantly influence clinical management strategies. Understanding these differences is essential for appropriate medication selection, monitoring protocols, and discontinuation planning in clinical practice.
Benzodiazepines represent the traditional class of sleep medications with well-established efficacy but significant dependency potential due to their broad effects on GABA-A receptor subtypes throughout the central nervous system. These medications produce not only sedative effects but also anxiolytic, muscle relaxant, and anticonvulsant properties that contribute to their therapeutic appeal while simultaneously increasing dependency risk. The dependency profile of benzodiazepines is characterized by rapid tolerance development, particularly to sedative effects, and severe withdrawal syndromes that can include life-threatening complications such as seizures and delirium tremens.
Non-benzodiazepine hypnotics, commonly referred to as Z-drugs, were developed to provide sleep-promoting effects with reduced dependency potential compared to traditional benzodiazepines. These medications demonstrate greater selectivity for specific GABA-A receptor subtypes that mediate sedation while having less effect on subtypes responsible for anxiolytic and muscle relaxant effects. However, clinical experience has revealed that Z-drugs still carry substantial dependency risk, particularly with long-term use, though withdrawal syndromes tend to be less severe than those associated with benzodiazepines.
The dependency profile of Z-drugs is characterized by gradual tolerance development that may be less apparent to patients and clinicians compared to benzodiazepines, potentially leading to inadvertent long-term use without recognition of developing dependency. Withdrawal from Z-drugs typically produces rebound insomnia and anxiety symptoms but rarely involves the severe medical complications associated with benzodiazepine withdrawal.
Dual orexin receptor antagonists represent a newer class of sleep medications that target the orexin system rather than GABA-ergic neurotransmission. These medications demonstrate significantly reduced dependency potential in clinical studies, with discontinuation studies showing minimal withdrawal symptoms or rebound phenomena. The reduced dependency risk appears related to their specific mechanism of action, which blocks wake-promoting signals rather than broadly enhancing inhibitory neurotransmission.
Melatonin receptor agonists constitute another class of sleep medications with minimal dependency potential due to their physiological mechanism of action that mimics endogenous circadian signaling. These medications rarely produce tolerance or withdrawal symptoms, making them attractive options for long-term use in appropriate patients. However, their efficacy may be limited compared to GABA-ergic medications, particularly for patients with severe insomnia.
Antidepressants used for sleep promotion, particularly trazodone and doxepin, present unique dependency considerations. While these medications do not typically produce the classic dependency syndrome associated with GABA-ergic hypnotics, discontinuation can result in antidepressant withdrawal symptoms that may be mistaken for sleep medication dependency. The sedating effects of these medications may also lead to psychological dependence despite the absence of significant physiological dependency.
Antihistamines, both prescription and over-the-counter formulations, are commonly used for sleep promotion and generally carry low dependency risk from a physiological perspective. However, tolerance to sedative effects develops rapidly, often leading to dose escalation and potential psychological dependence. Withdrawal from antihistamines rarely produces severe symptoms but may result in rebound insomnia and increased histamine-mediated symptoms.
Tolerance Mechanisms and Adaptive Responses
The development of tolerance to sleep medications represents a complex neuroadaptive process that fundamentally alters the brain’s response to pharmacological intervention and creates conditions that perpetuate medication dependence. Understanding tolerance mechanisms is crucial for clinicians managing patients with chronic insomnia and developing strategies to prevent or minimize dependency development.
Pharmacokinetic tolerance involves changes in drug absorption, distribution, metabolism, and elimination that reduce the effective concentration of medication at target sites. Chronic exposure to sleep medications can induce hepatic enzyme systems responsible for drug metabolism, leading to more rapid drug clearance and reduced therapeutic duration. This type of tolerance typically develops gradually and may be less apparent to patients who notice primarily that medication effects seem to wear off more quickly rather than becoming less effective overall.
Pharmacodynamic tolerance encompasses the more significant adaptive changes that occur at the cellular and molecular level in response to chronic medication exposure. These changes directly affect the brain’s response to medication regardless of drug concentration and represent the primary mechanism underlying clinically significant tolerance development. The complexity of pharmacodynamic tolerance involves multiple interconnected processes that occur simultaneously across different neural systems.
Receptor desensitization represents the most immediate adaptive response to chronic medication exposure, involving conformational changes in receptor proteins that reduce their sensitivity to both endogenous and exogenous ligands. For GABA-A receptors targeted by most sleep medications, desensitization occurs through phosphorylation of receptor subunits and uncoupling of receptor binding from ion channel activation. This process can begin within hours of initial medication exposure and contributes to acute tolerance phenomena.
Receptor downregulation involves the actual reduction in receptor number through decreased synthesis and increased degradation of receptor proteins. This adaptation requires longer timeframes to develop but produces more persistent tolerance that may require extended periods to reverse following medication discontinuation. The magnitude and distribution of receptor downregulation varies among different brain regions, contributing to the complex pattern of tolerance development that affects various aspects of medication response differently.
Compensatory upregulation of opposing neurotransmitter systems represents a crucial adaptation that maintains functional balance during chronic medication exposure but creates significant challenges during discontinuation. Enhanced glutamatergic neurotransmission develops to counteract chronic GABA-ergic enhancement, involving increased glutamate release, enhanced receptor sensitivity, and expanded receptor populations. These adaptations remain active during early discontinuation phases, creating the neurochemical basis for withdrawal symptoms and rebound phenomena.
Neuroplasticity changes extend beyond immediate receptor adaptations to encompass structural and functional alterations in neural circuits involved in sleep regulation. Chronic hypnotic exposure can alter dendritic morphology, synaptic strength, and connectivity patterns within sleep-wake regulatory networks. These changes may contribute to persistent sleep difficulties that outlast the period of acute withdrawal and require extended recovery periods.
Epigenetic modifications provide another layer of adaptive response that can produce long-lasting changes in gene expression patterns affecting neurotransmitter synthesis, receptor production, and neural development. These modifications may explain why some individuals experience protracted recovery periods following sleep medication discontinuation and why vulnerability to dependency development varies significantly among patients.
The temporal course of tolerance development varies considerably among different medication classes and individual patients. Short-acting medications may produce more rapid acute tolerance due to the repeated cycle of drug effect and elimination, while longer-acting medications may produce more gradual but potentially more profound adaptive changes. Individual factors including age, genetic polymorphisms, concurrent medications, and underlying medical conditions significantly influence tolerance development patterns.
Withdrawal Phenomena and Clinical Manifestations
Withdrawal from sleep medications encompasses a complex spectrum of physiological and psychological symptoms that can significantly impact patient well-being and complicate discontinuation efforts. The severity, duration, and specific characteristics of withdrawal phenomena vary considerably among different medication classes, individual patient factors, and discontinuation approaches, requiring individualized management strategies.
Acute withdrawal symptoms typically emerge within hours to days following medication discontinuation or dose reduction, depending on the pharmacokinetic properties of the specific medication. For short-acting medications, withdrawal symptoms may begin within hours of the last dose, while longer-acting medications may allow several days before symptoms become apparent. The intensity of acute withdrawal generally peaks within the first week following discontinuation and gradually subsides over subsequent weeks.
Rebound insomnia represents the most common and distressing withdrawal symptom, characterized by sleep difficulties that exceed the severity of pre-treatment insomnia. This phenomenon results from the compensatory neural adaptations that occurred during chronic medication use, particularly the upregulation of excitatory neurotransmission that becomes unopposed following medication removal. Rebound insomnia can be so severe that patients experience complete sleeplessness for several consecutive nights, creating significant distress and strong motivation to resume medication use.
Anxiety symptoms during withdrawal encompass both generalized anxiety and panic-like episodes that may be more severe than any pre-existing anxiety disorders. These symptoms reflect both the direct effects of removing GABA-ergic enhancement and the psychological distress associated with sleep disruption and fear of withdrawal progression. Anxiety symptoms can persist for weeks following discontinuation and may require specific therapeutic interventions to prevent relapse to medication use.
Physical withdrawal symptoms include tremor, muscle tension, headaches, nausea, and in severe cases involving benzodiazepines, potentially life-threatening complications such as seizures. The severity of physical symptoms correlates with the degree of neuroadaptation that occurred during chronic use and the rapidity of discontinuation. Gradual tapering protocols significantly reduce the risk of severe physical withdrawal symptoms but may prolong the overall duration of the withdrawal process.
Cognitive symptoms during withdrawal include concentration difficulties, memory problems, and subjective experiences of mental cloudiness or confusion. These symptoms can significantly impact daily functioning and may persist for extended periods following acute withdrawal resolution. The cognitive effects of withdrawal may be particularly problematic for individuals whose work or personal responsibilities require high levels of mental performance.
Perceptual disturbances represent less common but highly distressing withdrawal symptoms that can include increased sensitivity to light and sound, visual distortions, and in severe cases, hallucinations. These symptoms are more commonly associated with benzodiazepine withdrawal but can occur with other medication classes, particularly when discontinuation is abrupt or when high doses were previously used.
Protracted withdrawal syndrome involves the persistence of withdrawal symptoms for months or even years following acute withdrawal resolution. This phenomenon is not well understood but may involve long-lasting neuroadaptive changes or epigenetic modifications that require extended periods to resolve. Protracted withdrawal can include persistent sleep difficulties, anxiety, cognitive impairment, and mood disturbances that significantly impact quality of life.
The psychological component of withdrawal encompasses not only the direct neurochemical effects of medication removal but also the psychological distress associated with losing a reliable sleep aid. Many patients develop significant anxiety about their ability to sleep without medication, creating anticipatory anxiety that can worsen sleep difficulties and perpetuate the cycle of medication dependence.
Individual factors that influence withdrawal severity include duration and dose of previous medication use, rate of discontinuation, concurrent medical and psychiatric conditions, social support systems, and previous experiences with medication withdrawal. Patients with longer duration of use and higher doses typically experience more severe withdrawal, while those with strong social support and comprehensive medical management tend to have better outcomes.
Psychological Dependency and Behavioral Patterns
Psychological dependency on sleep medications represents a complex phenomenon that extends beyond physiological adaptation to encompass behavioral, cognitive, and emotional patterns that perpetuate medication reliance. Understanding these psychological dimensions is essential for developing comprehensive treatment approaches that address both the physiological and psychological aspects of dependency.
Sleep-related anxiety represents a central component of psychological dependency, involving persistent worry about the ability to sleep without medication assistance. This anxiety often develops gradually as patients begin to question whether their natural sleep mechanisms remain functional after periods of medication use. The resulting fear of sleeplessness can create anticipatory anxiety that actually impairs sleep, creating a self-fulfilling prophecy that reinforces the perceived need for medication.
Catastrophic thinking patterns commonly develop among individuals with psychological dependency on sleep medications, involving excessive worry about the consequences of poor sleep and overestimation of the negative impact of sleep loss. These cognitive distortions contribute to medication reliance by creating perceived necessity for pharmaceutical intervention to prevent imagined catastrophic outcomes from sleep deprivation.
Behavioral conditioning plays a significant role in psychological dependency through the repeated pairing of medication use with sleep initiation. Over time, the act of taking medication becomes a conditioned stimulus that triggers relaxation and sleep preparation responses, while the absence of this ritual creates anxiety and alertness that interfere with natural sleep processes. This conditioning can persist long after physiological dependency has resolved.
Loss of sleep self-efficacy represents another crucial psychological factor, involving diminished confidence in one’s natural ability to initiate and maintain sleep without pharmaceutical assistance. This loss of confidence often develops gradually as individuals become increasingly reliant on medication for sleep and lose touch with their natural sleep regulatory mechanisms. Rebuilding sleep self-efficacy becomes an essential component of successful medication discontinuation.
Medication serves multiple psychological functions beyond sleep promotion that contribute to dependency development. Many individuals use sleep medications to manage anxiety, stress, or emotional distress, creating psychological reliance that extends beyond their primary sleep-promoting effects. The medication may become associated with feelings of control, security, and emotional regulation that are difficult to replace through alternative means.
Avoidance behaviors commonly develop as individuals become increasingly dependent on medication for sleep, including avoidance of situations that might disrupt sleep routines or create anxiety about sleep. These behaviors can limit lifestyle flexibility and create additional psychological pressure to maintain medication use to preserve established routines and avoid feared consequences.
Social and occupational pressures often contribute to psychological dependency by creating perceived necessity for reliable sleep to meet performance demands. Individuals in high-responsibility positions may feel unable to risk the sleep disruption associated with medication discontinuation, creating psychological barriers to attempting withdrawal even when motivated to reduce medication dependence.
The development of psychological dependency is influenced by individual factors including personality traits, coping strategies, previous experiences with sleep difficulties, and underlying mental health conditions. Individuals with anxiety disorders, perfectionist tendencies, or poor stress management skills may be particularly vulnerable to developing psychological dependency on sleep medications.
Cultural and societal attitudes toward sleep and medication use also influence psychological dependency development. In cultures that emphasize productivity and performance, sleep difficulties may be viewed as unacceptable problems requiring immediate pharmaceutical solution, contributing to psychological pressure to maintain medication use rather than addressing underlying factors through lifestyle or behavioral modifications.
Consequences of Long-Term Dependency
The consequences of long-term sleep medication dependency extend far beyond the immediate effects of withdrawal to encompass multiple domains of health and functioning that can significantly impact quality of life and overall well-being. Understanding these consequences is crucial for both patients and healthcare providers making decisions about long-term hypnotic use and discontinuation strategies.
Cognitive function deterioration represents one of the most significant long-term consequences of chronic sleep medication use, particularly with medications that broadly affect GABA-ergic neurotransmission. Chronic use of benzodiazepines and related compounds has been associated with progressive decline in memory formation, attention, and executive function that may persist even after medication discontinuation. These cognitive effects can impact occupational performance, interpersonal relationships, and overall life satisfaction.
The relationship between chronic hypnotic use and dementia risk has become an area of increasing concern based on epidemiological studies suggesting associations between long-term benzodiazepine use and increased dementia incidence. While causality remains debated, the potential for irreversible cognitive decline represents a significant consideration in risk-benefit analyses of long-term sleep medication use, particularly in older adults who may be more vulnerable to cognitive adverse effects.
Sleep architecture disturbances persist throughout chronic medication use and can contribute to non-restorative sleep despite adequate sleep duration. Many sleep medications suppress deep sleep stages and REM sleep that are essential for physical restoration and memory consolidation. These alterations in sleep quality may contribute to daytime fatigue, mood disturbances, and cognitive impairment that paradoxically worsen the problems that sleep medications were intended to address.
Physical health consequences of long-term sleep medication dependency include increased fall risk, particularly in older adults, due to residual sedation and impaired balance and coordination. The risk of serious injury from falls can significantly impact morbidity and mortality, making the safety profile of long-term hypnotic use a critical consideration in vulnerable populations.
Respiratory depression represents a potentially serious consequence of chronic sleep medication use, particularly when combined with other central nervous system depressants or in individuals with underlying respiratory conditions. The cumulative effects of chronic medication use may increase vulnerability to respiratory complications that could prove life-threatening in certain circumstances.
Tolerance development necessitates progressive dose escalation to maintain therapeutic effects, creating a cycle of increasing medication exposure and potentially greater adverse effects. The need for higher doses over time can lead to medication regimens that exceed recommended guidelines and increase the risk of serious adverse events while providing diminishing therapeutic benefit.
Social and occupational consequences of sleep medication dependency can include stigma associated with medication dependence, limitations on activities due to sedation or cognitive impairment, and conflicts with family members or healthcare providers regarding medication use. These social consequences can contribute to psychological distress and complicate efforts to address dependency issues.
Financial costs associated with long-term sleep medication use can become substantial over time, particularly for newer medications that may not have generic alternatives. The economic burden extends beyond medication costs to include healthcare utilization for dependency-related problems and potential productivity losses due to medication-related impairment.
Healthcare utilization patterns often change with long-term dependency, including increased emergency department visits for sleep-related crises, multiple provider consultations seeking prescription renewals, and complex medical management of withdrawal attempts. These patterns can strain healthcare resources and create challenges for coordinated care delivery.
Legal and regulatory consequences may arise for individuals who develop tolerance requiring doses that exceed prescribed amounts, potentially leading to medication-seeking behaviors that could have legal implications. Healthcare providers may also face regulatory scrutiny for prescribing patterns that suggest inadequate monitoring of long-term hypnotic use.
The impact on family relationships can be significant as dependency affects not only the individual but also family members who may be concerned about medication use, frustrated by failed attempts at discontinuation, or affected by changes in the dependent individual’s behavior and functioning. These relationship strains can create additional stress that complicates recovery efforts.
Evidence-Based Discontinuation Strategies
Successful discontinuation of sleep medications requires comprehensive, evidence-based approaches that address both the physiological and psychological aspects of dependency while minimizing withdrawal distress and preventing relapse. Contemporary research has established several key principles that should guide discontinuation efforts across different medication classes and patient populations.
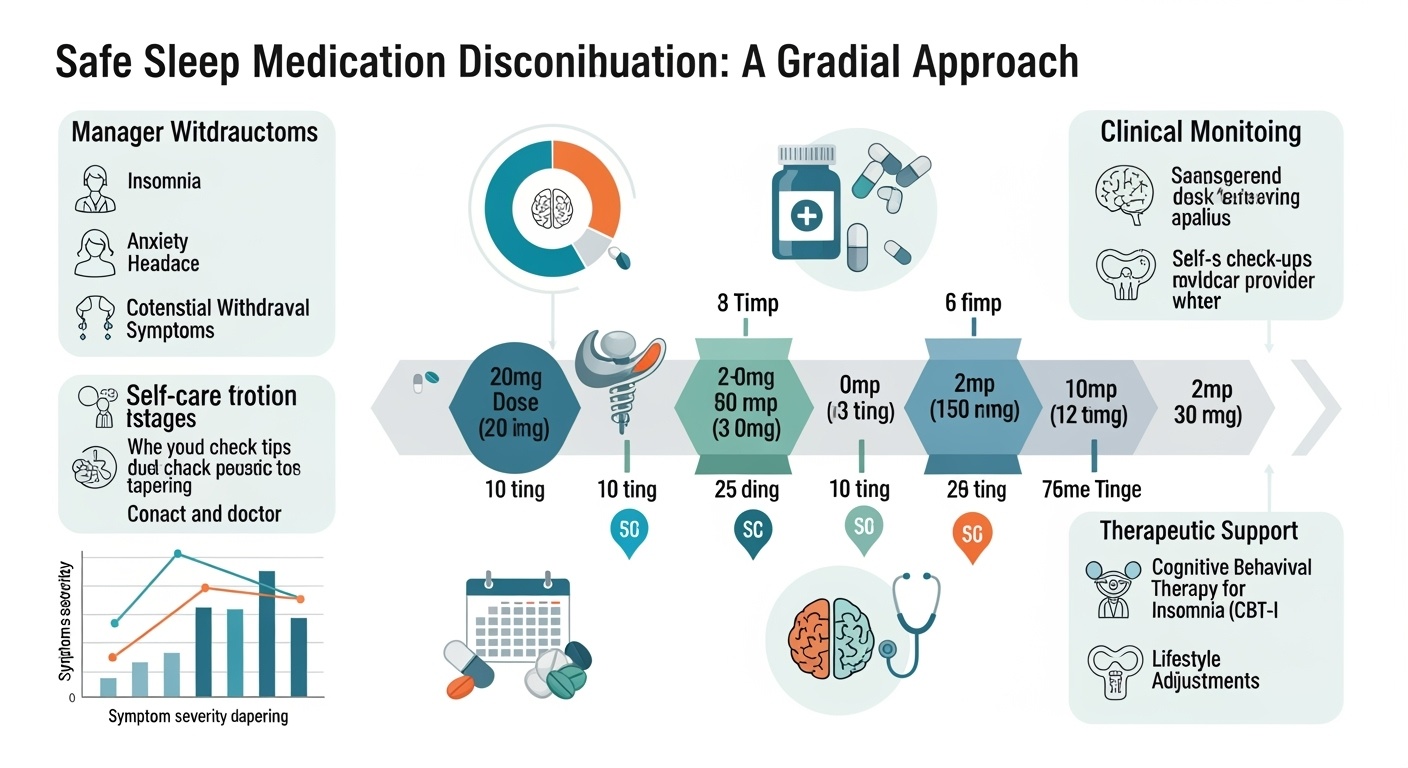
Gradual tapering represents the fundamental strategy for safe medication discontinuation, with research consistently demonstrating that slow, systematic dose reduction significantly reduces withdrawal severity compared to abrupt discontinuation. The optimal tapering schedule varies among medication classes, with benzodiazepines typically requiring reductions of ten to twenty-five percent every one to two weeks, while newer medications may allow somewhat more rapid reductions depending on individual patient response.
The tapering process should be individualized based on multiple factors including the specific medication, duration of use, starting dose, patient age, concurrent medical conditions, and previous withdrawal experiences. Patients who have been using higher doses for longer periods typically require more conservative tapering schedules, while those with shorter exposure periods may tolerate more rapid reductions.
Cross-tapering strategies involve substituting longer-acting medications for shorter-acting ones to provide more stable blood levels during the withdrawal process. This approach is particularly useful for patients taking short-acting benzodiazepines or Z-drugs, where substitution with longer-acting agents can reduce the severity of inter-dose withdrawal and provide greater control over the tapering process.
Cognitive behavioral therapy for insomnia represents an essential component of comprehensive discontinuation strategies, providing patients with evidence-based techniques for managing sleep difficulties without medication. CBT-I should ideally be initiated before or concurrent with medication tapering to ensure that patients have alternative strategies available when medication effects diminish. The combination of CBT-I with gradual tapering has been shown to improve both short-term withdrawal outcomes and long-term maintenance of medication-free sleep.
Sleep hygiene education forms a fundamental component of non-pharmacological approaches to sleep management during medication discontinuation. Patients should receive comprehensive instruction in sleep-promoting behaviors including consistent sleep schedules, appropriate sleep environment optimization, limitation of sleep-disrupting substances, and establishment of relaxing bedtime routines that can help compensate for reduced medication effects.
Adjunctive medications may be beneficial during the withdrawal process to manage specific symptoms and reduce withdrawal distress. These may include short-term use of medications for anxiety management, sleep aids with different mechanisms of action, or medications to address specific withdrawal symptoms such as nausea or headaches. The use of adjunctive medications should be carefully planned to avoid substituting one dependency for another.
Psychological support and counseling are crucial components of successful discontinuation, addressing the anxiety, fear, and behavioral patterns associated with sleep medication dependency. This support may include individual therapy, support groups, or intensive outpatient programs designed specifically for medication dependency issues. The psychological component of treatment should address not only withdrawal symptoms but also underlying factors that contributed to initial medication dependence.
Medical monitoring throughout the discontinuation process ensures early identification and management of complications while providing reassurance and support for patients experiencing withdrawal distress. Monitoring should include regular assessment of withdrawal symptoms, sleep quality, mood changes, and functional status, with adjustments to the tapering schedule based on patient response and tolerance.
Patient education regarding withdrawal expectations helps reduce anxiety and improve compliance with discontinuation protocols. Patients should understand that some degree of sleep disruption is normal during withdrawal, that symptoms are typically temporary, and that gradual improvement can be expected with appropriate management. Realistic expectations regarding the timeline for recovery help prevent premature abandonment of discontinuation efforts.
Emergency protocols should be established for managing severe withdrawal symptoms or complications that may arise during discontinuation. Patients and their families should understand when to seek immediate medical attention, and healthcare providers should be prepared to manage withdrawal emergencies including seizures, severe anxiety, or suicidal ideation that may occur during the withdrawal process.
Clinical Management Protocols
Effective clinical management of sleep medication discontinuation requires systematic approaches that integrate medical oversight, psychological support, and comprehensive care coordination to optimize patient outcomes while minimizing risks associated with withdrawal. These protocols should be tailored to individual patient needs while incorporating evidence-based best practices for safe discontinuation.
Initial assessment forms the foundation of successful discontinuation management, requiring comprehensive evaluation of medication history, dependency severity, withdrawal risk factors, and patient readiness for change. This assessment should include detailed documentation of current and historical medication use, previous withdrawal attempts, concurrent medical and psychiatric conditions, social support systems, and patient goals and expectations regarding discontinuation.
Risk stratification helps identify patients who may require more intensive monitoring or specialized interventions during discontinuation. High-risk patients include those with histories of seizures, severe psychiatric conditions, concurrent substance use disorders, or previous complicated withdrawal experiences. These patients may require inpatient or intensive outpatient management rather than routine office-based tapering protocols.
Collaborative treatment planning involves patients as active participants in developing discontinuation strategies that align with their individual circumstances, preferences, and goals. This collaborative approach improves treatment adherence and outcomes by ensuring that patients understand and agree with the proposed discontinuation plan while feeling empowered to communicate concerns or difficulties that arise during implementation.
Medication management protocols should specify exact tapering schedules, contingency plans for managing breakthrough symptoms, and criteria for adjusting tapering rates based on patient response. These protocols should include provisions for temporary stabilization at intermediate doses if withdrawal symptoms become severe, as well as guidelines for resuming previous doses if serious complications arise.
Monitoring schedules should be established based on withdrawal risk level and patient needs, with high-risk patients requiring more frequent contact and assessment. Monitoring should include systematic evaluation of withdrawal symptoms using validated assessment tools, sleep quality measures, mood and anxiety ratings, and functional status indicators. Regular monitoring allows for early intervention when problems arise and provides ongoing support throughout the discontinuation process.
Crisis management protocols are essential for addressing severe withdrawal symptoms or psychiatric emergencies that may occur during discontinuation. These protocols should specify criteria for immediate medical evaluation, emergency medication management, and coordination with psychiatric services when indicated. Healthcare providers should be prepared to manage complications including severe anxiety, panic attacks, psychosis, or suicidal ideation.
Integration with mental health services is crucial for patients with concurrent psychiatric conditions or those who develop significant psychological symptoms during withdrawal. Coordination with psychiatrists, psychologists, and other mental health professionals ensures comprehensive treatment that addresses both withdrawal management and underlying mental health issues that may complicate recovery.
Family involvement and education can significantly improve discontinuation outcomes by providing support systems and ensuring that family members understand the withdrawal process and their role in supporting recovery. Family education should include information about withdrawal symptoms, ways to provide support, warning signs requiring medical attention, and strategies for maintaining a supportive environment during recovery.
Documentation requirements should specify the information that must be recorded throughout the discontinuation process, including symptom assessments, medication changes, adverse events, and patient responses to interventions. Comprehensive documentation supports continuity of care, facilitates communication among providers, and provides legal protection for healthcare providers managing complex withdrawal cases.
Quality assurance measures should be implemented to ensure that discontinuation protocols are followed consistently and effectively. These measures may include regular review of patient outcomes, provider training updates, and systematic evaluation of protocol effectiveness with modifications based on clinical experience and emerging research evidence.
Pharmacological Interventions During Withdrawal
Pharmacological support during sleep medication withdrawal can significantly improve patient comfort and success rates while reducing the risk of severe complications. However, the use of adjunctive medications must be carefully planned to avoid creating new dependencies while providing appropriate symptomatic relief during the withdrawal process.
Anticonvulsants represent an important class of medications for managing withdrawal symptoms, particularly for patients discontinuing benzodiazepines who face seizure risk. Gabapentin and pregabalin have demonstrated efficacy in reducing withdrawal anxiety, sleep disturbances, and other autonomic symptoms while providing seizure prophylaxis. These medications offer the advantage of minimal dependency potential while effectively managing multiple withdrawal symptoms through their effects on calcium channels and GABA-ergic neurotransmission.
The dosing and duration of anticonvulsant therapy should be carefully planned to provide symptom relief during acute withdrawal while avoiding prolonged use that could create new dependencies. Gabapentin doses of three hundred to six hundred milligrams three times daily typically provide effective symptom management, with gradual tapering once acute withdrawal symptoms resolve. Pregabalin may be used at lower doses due to its greater potency and longer duration of action.
Beta-blockers can provide valuable support for managing cardiovascular symptoms of withdrawal including elevated heart rate, blood pressure, and palpitations. Propranolol is commonly used for this indication due to its central nervous system penetration and effectiveness in reducing both physical and psychological symptoms of anxiety. The typical dose range is twenty to forty milligrams two to three times daily, with monitoring for hypotension and other cardiovascular effects.
Alpha-2 agonists such as clonidine offer another approach to managing autonomic withdrawal symptoms through their effects on central nervous system arousal and sympathetic nervous system activity. Clonidine can be particularly helpful for managing sleep disturbances, anxiety, and physical discomfort during withdrawal. However, its use requires careful monitoring for hypotension and rebound hypertension if discontinued abruptly.
Buspirone represents a non-benzodiazepine anxiolytic that can provide anxiety relief during withdrawal without risk of cross-tolerance or dependency. Its delayed onset of action requires initiation several weeks before anticipated peak withdrawal symptoms, and its effectiveness may be limited in patients with severe anxiety or those previously exposed to benzodiazepines. Typical dosing ranges from fifteen to thirty milligrams daily in divided doses.
Antidepressants may be beneficial for patients with concurrent depression or those who develop significant mood symptoms during withdrawal. Selective serotonin reuptake inhibitors are generally preferred due to their favorable safety profile and lack of dependency potential. However, their delayed onset of action limits their utility for acute withdrawal management, and their use should be considered part of longer-term recovery planning rather than immediate symptom management.
Melatonin and melatonin receptor agonists can provide valuable support for sleep disturbances during withdrawal while promoting natural circadian rhythm regulation. These medications have minimal dependency potential and can be used safely for extended periods if needed. Melatonin doses of three to nine milligrams at bedtime are typically effective, while ramelteon may provide more sustained sleep support through its selective receptor activity.
Antihistamines may provide short-term relief for sleep disturbances and anxiety during withdrawal, though their anticholinergic effects and potential for tolerance limit their utility for extended use. Hydroxyzine is commonly used for its anxiolytic and sedating properties without dependency risk, though its anticholinergic effects may be problematic in older adults or those with certain medical conditions.
The timing and sequencing of adjunctive medications require careful consideration to optimize effectiveness while minimizing complexity and potential interactions. Some medications may be most beneficial when initiated before tapering begins, while others are best reserved for managing acute withdrawal symptoms. The goal is to provide adequate symptom relief while maintaining focus on the ultimate objective of medication-free sleep management.
Monitoring for interactions between adjunctive medications and tapering hypnotics is essential to ensure safety and effectiveness. Some combinations may potentiate sedation or other effects, requiring dose adjustments or timing modifications. Regular assessment of medication effectiveness and adverse effects allows for optimization of the pharmacological support regimen throughout the withdrawal process.
Non-Pharmacological Interventions
Non-pharmacological interventions form the cornerstone of sustainable recovery from sleep medication dependency, providing patients with evidence-based strategies for managing sleep difficulties and associated symptoms without reliance on pharmaceutical agents. These interventions must be implemented systematically and maintained long-term to support successful medication discontinuation and prevent relapse.
Cognitive behavioral therapy for insomnia represents the gold standard non-pharmacological intervention for sleep difficulties, with extensive research demonstrating its effectiveness both as a standalone treatment and as an adjunct to medication tapering. CBT-I addresses the cognitive and behavioral factors that perpetuate insomnia while teaching specific techniques for improving sleep quality and duration. The therapy typically involves four to eight sessions covering sleep education, stimulus control, sleep restriction, cognitive restructuring, and relapse prevention.
Sleep restriction therapy, a core component of CBT-I, involves temporarily limiting time in bed to match actual sleep time, thereby increasing sleep efficiency and reducing the time spent lying awake. This technique can be particularly challenging during medication withdrawal when sleep may be further disrupted, requiring careful titration and patient support to maintain adherence. The temporary sleep deprivation induced by sleep restriction helps consolidate sleep and rebuild natural sleep drive that may have been compromised by chronic medication use.
Stimulus control instructions help patients re-establish the bedroom environment as a strong cue for sleep rather than anxiety or wakefulness. These instructions include using the bed only for sleep and sexual activity, leaving the bedroom if unable to fall asleep within fifteen to twenty minutes, and maintaining consistent wake times regardless of sleep quality. Implementing stimulus control during withdrawal requires patience and persistence as patients work to overcome conditioned arousal responses to the sleep environment.
Cognitive restructuring addresses the dysfunctional beliefs and catastrophic thinking patterns that often develop around sleep and contribute to medication dependency. Patients learn to identify and challenge thoughts such as “I must have eight hours of sleep to function” or “I cannot sleep without medication,” replacing them with more realistic and helpful perspectives. This cognitive work is essential for reducing sleep-related anxiety and building confidence in natural sleep abilities.
Relaxation training encompasses various techniques including progressive muscle relaxation, deep breathing exercises, and mindfulness meditation that can help reduce physical and mental arousal that interferes with sleep. These techniques provide patients with active coping strategies for managing withdrawal symptoms and ongoing sleep difficulties. Regular practice is essential for developing proficiency and maximizing therapeutic benefit.
Mindfulness-based interventions have shown promise for addressing both sleep difficulties and the anxiety and emotional distress associated with medication withdrawal. Mindfulness techniques help patients observe withdrawal symptoms and sleep difficulties without becoming overwhelmed by them, reducing reactivity and promoting acceptance of temporary discomfort during recovery. These approaches can be particularly valuable for managing the psychological aspects of withdrawal.
Sleep hygiene education provides patients with comprehensive information about lifestyle factors that promote or interfere with sleep quality. This education covers topics including caffeine and alcohol use, exercise timing, light exposure, bedroom environment optimization, and pre-sleep routines. While sleep hygiene alone is rarely sufficient for treating chronic insomnia, it provides an important foundation for other interventions and helps optimize conditions for natural sleep.
Circadian rhythm regulation techniques help restore natural sleep-wake patterns that may have been disrupted by chronic medication use. These techniques include strategic light exposure, meal timing, and activity scheduling designed to strengthen circadian signals and promote appropriate timing of sleep and wakefulness. Bright light therapy in the morning and light restriction in the evening can be particularly helpful for patients whose circadian rhythms have become disrupted.
Exercise and physical activity programs can provide multiple benefits during medication withdrawal including improved sleep quality, reduced anxiety and depression, and enhanced overall physical health. Regular aerobic exercise has been shown to improve sleep onset, duration, and quality while reducing the need for sleep medications. However, exercise timing is important as vigorous activity close to bedtime can interfere with sleep.
Stress management and coping skills training help patients develop alternative strategies for managing the life stressors that may have originally contributed to sleep difficulties and medication dependence. These skills include problem-solving techniques, time management, communication skills, and stress reduction strategies that can reduce overall arousal and improve sleep quality.
Special Populations and Considerations
Certain patient populations require specialized approaches to sleep medication discontinuation due to unique physiological, psychological, or social factors that influence withdrawal risk and management strategies. Understanding these special considerations is essential for providing safe and effective care across diverse patient groups.
Older adults represent a particularly vulnerable population for sleep medication dependency and withdrawal complications due to age-related changes in drug metabolism, increased sensitivity to medication effects, and higher prevalence of concurrent medical conditions. Pharmacokinetic changes in older adults result in slower drug clearance and prolonged medication effects, increasing the risk of accumulation and toxicity during chronic use while potentially extending withdrawal duration.
The increased fall risk associated with sleep medications is particularly concerning in older adults, who may experience serious injuries from falls related to medication-induced sedation, confusion, or balance impairment. This risk persists during withdrawal as patients may experience rebound insomnia and daytime fatigue that contribute to cognitive impairment and increased fall risk. Withdrawal protocols for older adults typically require more conservative tapering schedules and enhanced safety monitoring.
Cognitive vulnerability in older adults may be exacerbated by both chronic medication use and withdrawal processes, requiring careful assessment and monitoring of cognitive function throughout discontinuation. The potential relationship between chronic benzodiazepine use and dementia risk makes successful discontinuation particularly important in this population, while also requiring specialized approaches that account for existing cognitive impairment.
Pregnant and breastfeeding women require specialized management due to potential effects of sleep medications on fetal development and infant health. Many sleep medications cross the placental barrier and can be transferred through breast milk, creating risks for developing fetuses and nursing infants. Discontinuation during pregnancy may be complicated by pregnancy-related sleep disturbances and concerns about withdrawal effects on both mother and fetus.
The timing of discontinuation relative to pregnancy planning requires careful consideration, as withdrawal during pregnancy may create additional stress and complications while continued medication use carries its own risks. Ideally, discontinuation should be completed before conception, but when this is not possible, specialized obstetric and psychiatric consultation is essential for developing safe management plans.
Patients with concurrent psychiatric disorders face unique challenges during sleep medication withdrawal due to the potential for psychiatric symptom exacerbation and increased risk of relapse to substance use or self-harm behaviors. Depression, anxiety disorders, bipolar disorder, and post-traumatic stress disorder can all be worsened by sleep disruption and withdrawal symptoms, requiring integrated treatment approaches that address both medication dependency and underlying psychiatric conditions.
The interaction between psychiatric medications and sleep medication withdrawal must be carefully managed to avoid dangerous drug interactions or psychiatric destabilization. Some psychiatric medications may help manage withdrawal symptoms, while others may complicate withdrawal or require dose adjustments during the tapering process. Coordination between sleep medicine specialists and psychiatrists is essential for optimal outcomes.
Patients with substance use disorders require specialized approaches due to increased risk of cross-addiction, complicated withdrawal syndromes, and potential interactions between sleep medication withdrawal and other substance use recovery programs. The principles of addiction treatment including motivation enhancement, relapse prevention, and long-term recovery support should be integrated into sleep medication discontinuation protocols.
Healthcare workers and shift workers face unique challenges related to their occupational demands and irregular sleep schedules that may have contributed to initial medication dependence. Discontinuation planning must account for work schedule demands while developing strategies for managing sleep difficulties in the context of shift work. Occupational health considerations may require coordination with employers or modification of work schedules during withdrawal.
Patients with chronic pain conditions often use sleep medications to manage both sleep difficulties and pain-related discomfort, creating complex interdependencies between pain management and sleep medication use. Withdrawal may temporarily worsen both sleep and pain symptoms, requiring integrated approaches that address both conditions simultaneously. Pain management specialists should be involved in treatment planning to ensure continuity of adequate pain control during withdrawal.
Patients with sleep disorders other than insomnia may have been inappropriately treated with hypnotic medications when specific sleep disorder treatments would have been more appropriate. These patients may require comprehensive sleep evaluation to identify and treat underlying sleep disorders such as sleep apnea, restless leg syndrome, or circadian rhythm disorders that contribute to sleep difficulties. Successful discontinuation may depend on addressing these underlying conditions.
Long-Term Recovery and Relapse Prevention
Successful long-term recovery from sleep medication dependency requires comprehensive strategies that extend well beyond the acute withdrawal period to address the ongoing challenges of maintaining medication-free sleep and preventing relapse to dependent medication use. Long-term recovery involves multiple interconnected components that must be sustained over time to achieve lasting freedom from sleep medication dependency.
Sleep skill maintenance represents a crucial component of long-term recovery, requiring patients to continue practicing and refining the behavioral and cognitive techniques learned during treatment. The skills acquired through cognitive behavioral therapy for insomnia and other non-pharmacological interventions require ongoing practice to maintain effectiveness and prevent deterioration over time. Patients should understand that these skills function similarly to physical fitness, requiring regular use to maintain strength and effectiveness.
Periodic booster sessions with sleep specialists or therapists can help reinforce learned skills, address emerging challenges, and prevent gradual drift away from effective sleep practices. These sessions may be scheduled at regular intervals during the first year following discontinuation, with frequency determined by individual patient needs and risk factors. Booster sessions provide opportunities to review sleep hygiene practices, update cognitive restructuring techniques, and address new stressors or challenges that may threaten sleep quality.
Relapse prevention planning involves identifying individual risk factors and developing specific strategies for managing situations that could lead to resumed medication use. Common relapse triggers include major life stressors, travel and schedule disruptions, illness, relationship problems, and work-related pressures. Patients should develop detailed action plans for managing these situations without resorting to sleep medications, including alternative coping strategies and emergency support resources.
Early warning sign recognition helps patients identify subtle changes in sleep patterns, mood, or behavior that may indicate increased risk for relapse. These warning signs might include gradually increasing time spent lying awake, growing anxiety about sleep, increasing use of alcohol or other substances to aid sleep, or recurring thoughts about the benefits of sleep medications. Recognition of these warning signs allows for early intervention before problems become severe.
Coping strategy refinement involves ongoing development and practice of alternative approaches to managing sleep difficulties and related stressors. Patients should continue to expand their repertoire of relaxation techniques, stress management strategies, and problem-solving skills that can be applied when sleep challenges arise. This ongoing skill development helps build confidence and resilience against future sleep difficulties.
Social support maintenance is essential for long-term recovery success, requiring patients to maintain connections with family members, friends, and healthcare providers who understand their recovery journey and can provide encouragement and assistance when needed. Support groups, whether formal or informal, can provide ongoing motivation and practical advice from others who have successfully maintained medication-free sleep.
Lifestyle optimization involves ongoing attention to factors that support natural sleep and overall health, including regular exercise, healthy diet, stress management, and maintenance of consistent sleep schedules. These lifestyle factors require long-term commitment and may need periodic adjustment as life circumstances change. Patients should understand that maintaining healthy lifestyle practices is an ongoing investment in their sleep quality and overall well-being.
Medical follow-up scheduling ensures regular assessment of sleep quality, overall health, and recovery maintenance over time. Follow-up appointments may become less frequent as recovery stabilizes, but periodic check-ins allow for early identification of problems and provide ongoing support and encouragement. These appointments also provide opportunities to address any new medical conditions or medications that might affect sleep.
Medication vigilance involves ongoing awareness of the dependency potential of any new medications prescribed for other conditions. Patients should inform all healthcare providers about their history of sleep medication dependency and request non-dependent alternatives when possible. This vigilance extends to over-the-counter medications and supplements that may have sedating effects or dependency potential.
Quality of life monitoring helps ensure that the benefits of medication-free sleep are maintained over time and that any deterioration in life satisfaction is addressed promptly. Regular assessment of mood, energy level, cognitive function, and overall life satisfaction can help identify emerging problems that might threaten recovery. This monitoring should include attention to both sleep-related and general quality of life measures.
Contingency planning for medical emergencies or situations requiring hospitalization helps ensure that sleep medication dependency history is communicated to healthcare providers and that appropriate precautions are taken if sedative medications are necessary for medical procedures. Patients should carry medical alert information and have clear preferences documented regarding medication use in emergency situations.
| Medication Class | Dependency Risk Level | Withdrawal Severity | Recommended Tapering Duration | Key Management Considerations |
| Benzodiazepines | High | Severe (potential life-threatening complications) | 8-16+ weeks depending on duration of use | Requires medical supervision, possible cross-tapering, seizure precautions |
| Non-Benzodiazepine Hypnotics (Z-drugs) | Moderate to High | Moderate to Severe | 4-12 weeks | Less severe than benzodiazepines but still requires gradual tapering |
| Dual Orexin Receptor Antagonists | Low | Mild | 2-4 weeks | Minimal withdrawal symptoms, can often taper more rapidly |
| Melatonin Receptor Agonists | Very Low | Minimal | 1-2 weeks | Rarely causes significant withdrawal symptoms |
| Sedating Antidepressants | Low to Moderate | Mild to Moderate | 4-8 weeks | May cause antidepressant discontinuation syndrome |
| Withdrawal Phase | Timeline | Primary Symptoms | Management Strategies | Clinical Priorities |
| Acute Withdrawal | 1-7 days post-discontinuation | Rebound insomnia, anxiety, physical symptoms | Symptom monitoring, adjunctive medications, sleep hygiene | Safety monitoring, symptom relief |
| Early Recovery | 1-4 weeks | Persistent sleep difficulties, mood symptoms | CBT-I implementation, lifestyle modifications | Skill building, psychological support |
| Stabilization | 1-3 months | Gradual sleep improvement, occasional setbacks | Continued therapy, relapse prevention planning | Long-term strategy development |
| Long-term Recovery | 3+ months | Maintenance of gains, vigilance for triggers | Booster sessions, ongoing support | Relapse prevention, quality of life |
| Risk Factor Category | Specific Risk Factors | Assessment Methods | Management Modifications |
| Patient Demographics | Age >65, female gender, concurrent medical conditions | Comprehensive medical history, functional assessment | Conservative tapering, enhanced monitoring |
| Medication Factors | High dose, long duration of use, multiple medications | Prescription history review, current medication assessment | Extended tapering period, cross-tapering consideration |
| Psychological Factors | Anxiety disorders, depression, substance use history | Psychiatric evaluation, psychological testing | Integrated mental health treatment, specialized counseling |
| Social Factors | Limited support systems, high-stress occupation | Social assessment, occupational evaluation | Enhanced support services, work accommodation planning |
| Medical Complexity | Multiple comorbidities, polypharmacy, previous withdrawal failures | Comprehensive medical evaluation, specialist consultation | Multidisciplinary team approach, specialized protocols |
Conclusions and Clinical Implications
Sleep medication dependency represents a complex clinical challenge that requires sophisticated understanding of neurobiological mechanisms, comprehensive assessment of individual risk factors, and implementation of evidence-based management strategies that address both physiological and psychological aspects of dependency. The growing recognition of this problem’s scope and significance has important implications for clinical practice, healthcare policy, and future research directions.
The neurobiological foundation of sleep medication dependency involves intricate adaptive processes that fundamentally alter brain function in ways that extend well beyond the original therapeutic targets. Understanding these mechanisms is essential for healthcare providers managing patients with chronic insomnia, as it informs both prevention strategies and treatment approaches. The recognition that different medication classes carry substantially different dependency risks should influence prescribing practices and patient education efforts.
Prevention of sleep medication dependency should be prioritized through careful patient selection, appropriate medication choice, regular monitoring, and early implementation of non-pharmacological interventions. Healthcare providers should approach sleep medication prescribing with clear understanding of dependency risks and commitment to time-limited use whenever possible. The integration of cognitive behavioral therapy for insomnia and other evidence-based non-pharmacological approaches should be standard practice rather than afterthought interventions.
Assessment and management of established dependency requires individualized approaches that account for medication-specific factors, patient characteristics, and social circumstances that influence withdrawal success. The evidence strongly supports gradual tapering protocols over abrupt discontinuation, with tapering schedules tailored to specific medications and individual patient responses. Healthcare providers must be prepared to manage withdrawal complications while providing comprehensive support throughout the discontinuation process.
The integration of pharmacological and non-pharmacological interventions offers the most promising approach to successful medication discontinuation, with cognitive behavioral therapy for insomnia serving as the cornerstone of sustainable recovery. Adjunctive medications can provide valuable symptom relief during withdrawal while non-pharmacological approaches build the foundation for long-term medication-free sleep management.
Special populations require modified approaches that account for unique physiological and psychosocial factors that influence dependency risk and withdrawal management. Older adults, pregnant women, patients with psychiatric comorbidities, and those with concurrent substance use disorders need specialized protocols that address their specific vulnerabilities and treatment needs.
Long-term recovery requires sustained commitment to behavioral changes, ongoing skill maintenance, and vigilance against relapse triggers. The success of discontinuation efforts should be measured not only by successful medication cessation but also by maintained sleep quality, functional improvement, and overall quality of life enhancement.
Healthcare system implications include the need for provider education regarding dependency risks and management strategies, development of systematic approaches to monitoring long-term hypnotic use, and integration of sleep medicine expertise into primary care and psychiatric settings. Healthcare policies should support access to evidence-based non-pharmacological treatments while promoting responsible prescribing practices for sleep medications.
Future research priorities should focus on developing more precise methods for predicting dependency risk, optimizing discontinuation protocols for different patient populations, and investigating novel therapeutic approaches that provide effective sleep promotion without dependency potential. The development of personalized medicine approaches based on genetic, neurobiological, and psychological factors could significantly improve both treatment selection and discontinuation outcomes.
The economic implications of sleep medication dependency extend beyond direct medication costs to encompass healthcare utilization, productivity losses, and social welfare impacts that justify investment in prevention and treatment programs. Healthcare systems should recognize that comprehensive dependency management, while initially resource-intensive, can produce long-term cost savings and improved population health outcomes.
In conclusion, sleep medication dependency represents a significant public health challenge that requires coordinated response from healthcare providers, policymakers, and researchers. The available evidence provides clear guidance for safe and effective management approaches, but successful implementation requires systemic changes in how sleep disorders are approached and treated. The ultimate goal should be optimization of sleep health through approaches that provide effective symptom relief while minimizing long-term dependency risks and promoting sustainable recovery for those who develop dependency. The complexity of this challenge demands ongoing commitment to evidence-based practice, patient-centered care, and continued research to refine and improve treatment approaches for this important clinical problem.
 mood and rest
mood and rest